Germline (GL) RUNX1 (RUNX1GL) mutations result in autosomal-dominant familial platelet disorder (FPD) and are associated with heightened risk of myeloid neoplasia (MN). Indeed about 43% of RUNX1GL carriers are expected to develop MN, by the age of 50 yrs. 1 AML progression can be accompanied by biallelic RUNX1 hits. The phenotypic heterogeneity, incomplete penetrance, and leukemogenic risk depend on mutational burden and functional impact (topology of mutations, configuration) of RUNX1 and thus evolution of MN may be delayed. 2-3
We hypothesized that a large systematic study of RUNX1GL including types and topology will elucidate the impact of various RUNX1mutations ( RUNX1MT), their configuration, and molecular associations to provide prognostic insights and mechanistic conclusions as to the pathogenesis of these mutations.
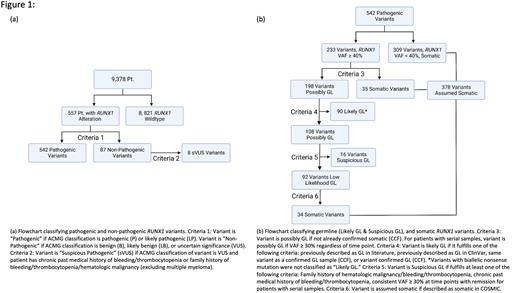
We analyzed over 9000 patients from Cleveland Clinic Foundation (CCF; n=7953) and public series. 4-5 We devised a novel rational algorithm to assess of RUNX1GL variants ( Fig1A). Stringent criteria were used that prioritized exclusion of false positives: GL benign and likely benign alterations were not included, while pathogenic and likely pathogenic (P/LP) variants were further curated. Carriers of VUS RUNX1MT that displayed past medical history or family history suspicious for FPD or leukemia were classified as VUS of suspicious pathogenicity (sVUS). The remaining variants, including P/LP, were further filtered and clinically annotated ( Fig1B). GL confirmation was possible in 22 alterations, e.g., wherein our algorithm resulted in ambivalent assignment.
We found 627 RUNX1 variants (GL and somatic) in 557 patients, of which 8 were sVUS and 106 (17%) were found to be GL P/LP including 3 new RUNX1GL alterations (Y281*, S141L, S410*).
The mean age at presentation of RUNX1GL carriers was lower (64 yrs.) compared to patients with somatic RUNX1MT(70 yrs., P= .0053). There was no difference in disease phenotype (AML, MDS, MDS/MPN) between GL, somatic, and biallelic (somatic/somatic) RUNX1MT carriers. Of note, 1 patient with predicted RUNX1GL variants had aplastic anemia. Cytogenetic analysis revealed that 68% of RUNX1GL cases were associated with normal karyotype. Four RUNX1GL cases also displayed trisomy 21. FLT3 ITD/PTDwas more prevalent in RUNX1GL carriers (14.8%) than in those with somatic RUNX1MT(4.7%) ( P= .021). PHF6 MT were more frequent in carriers of RUNX1GL (18%) compared to somatic RUNX1 carriers (9%) ( P= .078). Patients with a second RUNX1 hit included somatic biallelic (n=52)and GL/somatic (n=6) RUNX1 MT. The most common mutations co-occuring with biallelic RUNX1 hit werethose in EZH2 (27%, P= .025), ZRSR2 (19%, P= .020), U2AF1 (21%, P= .030) and PTPN11 (16%, P= .013) compared to monoallelic RUNX1MT.
We further categorized RUNX1MT according to their predicted functional effects as: i) haploinsufficient (RUNT-truncating mutations); ii) dominant negative (post-RUNT nonsense, C-terminal frameshift, and missense mutations previously described as dominant negative); and iii) RUNT missense mutations. 6-7 We analyzed 93 (25%) mutations classified as haploinsufficient, 98 (27%) as dominant negative, and 134 (36%) as RUNT missense. There was no difference in the topology between somatic and GL hits. Survival analysis indicated that there was no difference in prognosis between dominant negative, haploinsufficient, and RUNT missense mutations ( P= .59).
Among RUNX1GL carriers, there was also no prognostic difference between subtypes of RUNX1GL ( P= .896). To determine whether GL mutation types convey a greater penetrance, we compared the age of patients at presentation for somatic and GL. Specifically, carries of GL dominant negative mutations were diagnosed at a younger median age (60.5 yrs.) compared to carriers of GL haploinsufficient (68 yrs.) and RUNT missense (69 yrs.). However, this trend was not statistically significant. To rule out the effect of RUNX1GL lesions on genome instability, we quantified the mutational burden per each GL variant. Compared to RUNT missense, haploinsufficient GL variants were associated with a greater number of co-occurring mutations ( P= .013) while dominant negative GL variants were not ( P= .054).
In summary, our study of the topological features of RUNX1GL variants showed that dominant negative RUNX1 mutations are frequent in MN and are not associated with specific phenotypes, but rather define a variable penetrance.
Disclosures
Carraway:BMS: Consultancy, Research Funding, Speakers Bureau; Stemline Therapeutics: Consultancy, Speakers Bureau; Jazz Pharmaceuticals: Consultancy, Other: Travel, Accommodations, Expenses , Speakers Bureau; Agios: Consultancy, Speakers Bureau; Novartis: Consultancy, Other: Travel, Accommodations, Expenses , Speakers Bureau; Genentech: Consultancy; AbbVie: Other; Astex Pharmaceuticals: Other; Syndax: Other: DSMB; Takeda: Other; Daiichi: Consultancy; Celgene: Research Funding. Maciejewski:Novartis: Honoraria, Speakers Bureau; Regeneron: Consultancy, Honoraria; Alexion: Membership on an entity's Board of Directors or advisory committees; Omeros: Consultancy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal